Eliminating scaling of ultrafiltration membrane
Eliminating scaling of ultrafiltration membrane
Project Name
300 KLD Zero Liquid Discharge (ZLD) Plant at Ascent Yarns Pvt Ltd.
In capacity of Operations & Maintenance consultancy SWA Environment provided troubleshooting for frequent increase in feed pressure of ultrafiltration system which was a part of 300 MLD ZLD based treatment plant at Ascent Yarns Pvt Ltd.
Objective
To normalize the frequency of increase in feed pressure of the ultrafiltration system
Location
Ahmedabad, India
Project Description
Ascent Yarns was having a 300 KLD UF system as a part of its 300 MLD ZLD based treatment plant at its textile dyeing house.

300 KLD UF plant at Ascent Yarns Pvt. Ltd.

Working range of filtration processes
Solution:
PART 1 – Identifying and listing common probably cause for the problem
The first step in the methodology for the solution was to identify the common causes and eliminate each by logical explanation
Common Challenges:
- High TSS >250 mg/l (For MBR UF membranes it can sustain >12-16 g/l) Not a reason
- DHigh COD >150 mg/l Not a reason as the feed COD measured was <100 mg/l
- Improper cleaning (Backwash frequency, air scouring etc) Proper cleaning was witnessed
Typical/ Uncommon problems:
- Scaling due to higher Ca+2, SO4-2, Mg+2 etc. ions which are common in textile effluent
- Oil & Grease – Extremely detrimental to the membrane material Not a reason as measured was <2 mg/l
PART 2 – Detailing the reason identified
Scaling in Ultra Filtration
CAUSE
Precipitation due to increase in Ip-Ionic product compared to Ksp- Solubility product.
IMPACT
Clogging in membrane and very frequent pressure drop.
REMEDIES
- Removal of ions likely to participate in precipitation reaction. Mostly it is Calcium, Sulphate, Magnesium & Carbonate
- Removal can be done by ion exchange before the UF membrane.
PART 3 – Prepare a hypothesis explaining the mechanism and supporting it with water chemistry investigations
Step 1 Scaling mechanism through investigation of water quality parameters
Effluent characteristics after treatment in ETP (Biological + Coagulation + PSF + ACF )
- COD <50-70 mg/l
- TSS <20-50 mg/l
- Ca+2 – 50-500 mg/l (Higher when cotton dyeing is carried out)
- SO4-2 – 20-500 mg/l (Higher when cotton dyeing is carried out)
Note
Glauber’s salt which is Sodium sulphate & Calcium Chloride are common reagent used in cotton dyeing process
Step 2 Scaling mechanism through investigation water chemistry assessment

Thus, it will precipitate out from the solution.
BUT wait this is an overly simplistic and ideal condition calculation !!!!
Step 3 Provide a more accurate and representative model for the mechanism
Chemical equilibrium analysis in Visual Minteq was carried out (A great tool to avoid lengthy hand calculation and use of LogC-pH graphs)
Following are the screenshot of the model run which was carried out for this scenario
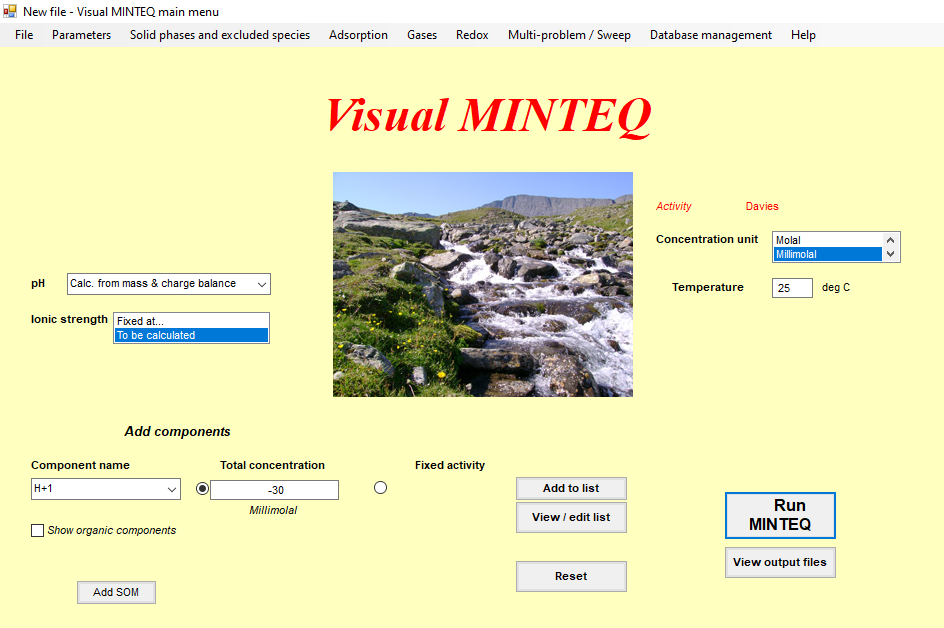
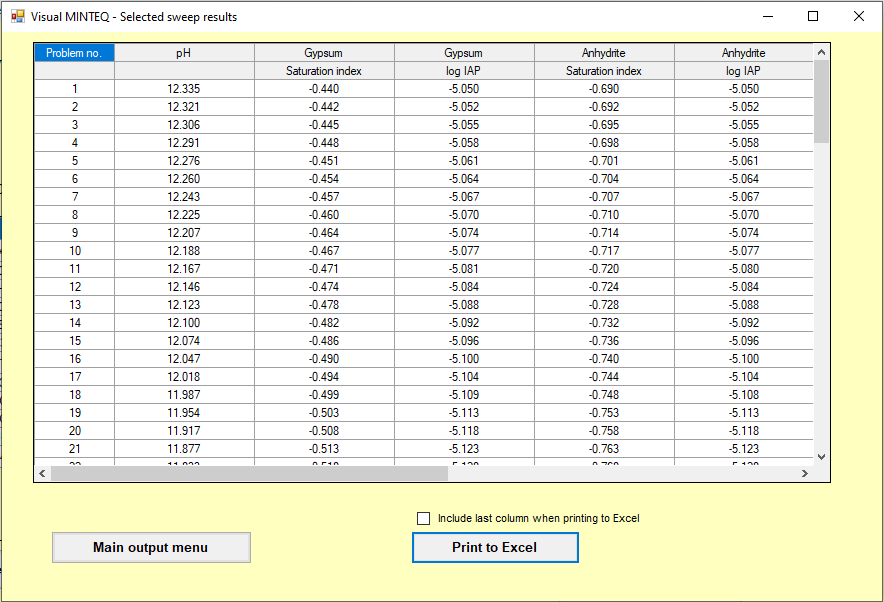
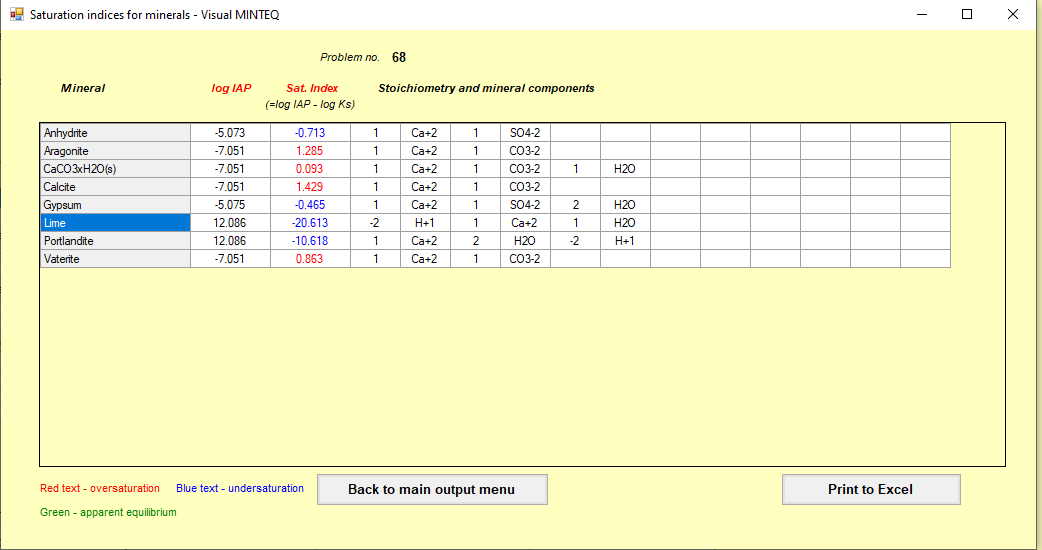
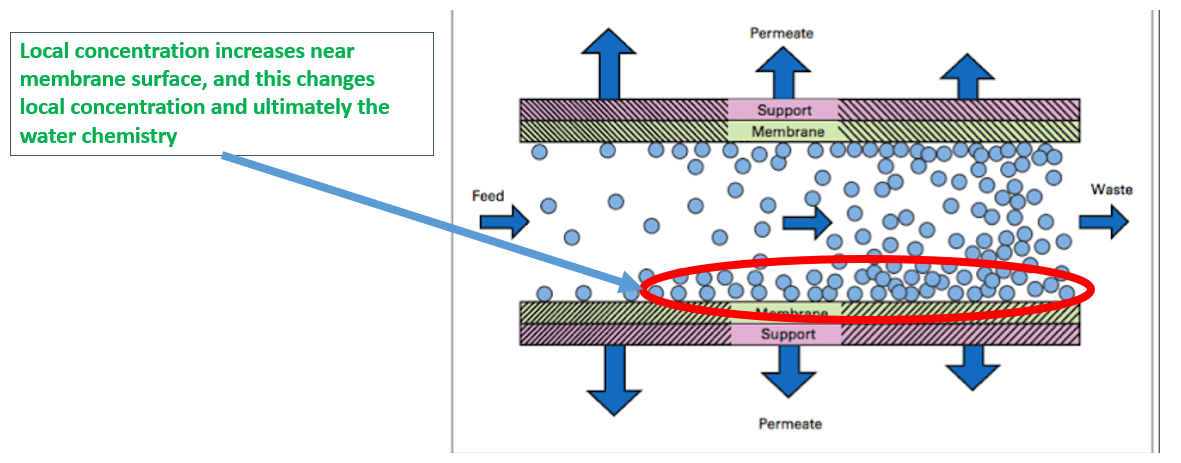
Step 4 Provide a physical explanation of the proposed mechanism
It was assumed that the precipitation was happening locally and the same can only be eliminated by removing either Calcium or Sulphate ions
PART 4 – Prove the hypothesis by piloting and practical trial.
The hypothesis was provided by deploying an existing ion exchange based softening plant between the UF and ETP. It was assumed that the softening plant will eliminate the Calcium ion and the sulphate will not have a favorable pairing with any ions which has a low solubility at membrane surface level.
This solution was applied, and the problem was eliminated immediately !
This problem was thus resolved without providing any capital investment and only by working on the good old fundamental water chemistry textbook principles. The solution highlights our core competency of strong process fundamentals and core philosophy to provide our clients with low cost solutions.
We thank our client Ascent Yarns Pvt Ltd. to be provide us an opportunity to work on this problem.
