SWA Enviro offers ISO 8573-7 microbial testing of compressed air for pharmaceutical, medical device, and food industries. Learn how to protect your cleanroom and ensure compliance.
Microbial testing of compressed air, specifically bioburden testing per ISO 8573-7, is a standard practice in the pharmaceutical, medical device, and food industries. This is because microbial contaminants in compressors or air lines can severely impact final products. A consistent micro-testing program can help detect potential issues early, preventing costly production shutdowns.
While most regulated manufacturing facilities routinely sample environmental air, compressed air and process gases are often overlooked as potential sources of microbial contamination. Specialized samplers, using contact plates, can effectively capture microorganisms in these air streams. Microbial testing of compressed air is crucial to prevent contamination within controlled environments like production areas and cleanrooms.
Microorganisms like bacteria, yeasts, molds, and viruses are a significant concern for compressed air systems. Millions of these enter the air system through the compressor intake because intake filters don’t remove viable particles. While modern, well-maintained compressor systems with proper filtration and point-of-use filters can remove these contaminants, a monitoring program is essential to ensure this protection is consistently safeguarding your products.
Why Test Compressed Air for Microbial Contamination?
Compressed air systems can harbor microbial contaminants from various sources:
- Ambient air intake: The air entering compressors can carry dust, spores, and bacteria.
- Moisture: Even with dryers and filters, small amounts of condensate can remain and support microbial growth.
- Pipework and fittings: Bacteria and fungi can colonize internal surfaces, especially if there are dead legs or areas with stagnant air.
- Maintenance activities: Poor hygiene during servicing can introduce contaminants.
👉 That’s why ISO 8573-7 and GMP (Good Manufacturing Practices) guidelines mandate regular microbial monitoring of compressed air in cleanrooms and critical production areas.
What Is ISO 8573-7 Microbial Testing?
ISO 8573-7 focuses on the biological (viable) contaminants in compressed air. It requires routine testing of compressed air that comes into direct or indirect contact with your product, equipment, or sterile zones.
Microbial Testing Methods We Use
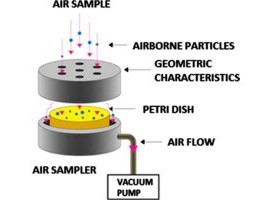
Impaction Sampling (e.g., with a microbial air sampler):
-
- Air is directed through a sampler onto an agar plate.
- The plate is incubated under controlled conditions to allow colonies to grow.
- Results are expressed as CFU/m³ (colony-forming units per cubic meter).
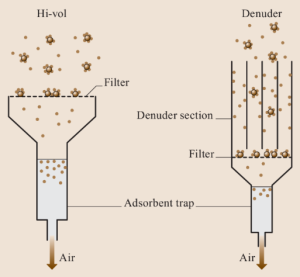
Filtration Sampling:
-
- Air passes through a sterile filter that traps microorganisms.
- The filter is then incubated or flushed with sterile fluid and cultured.
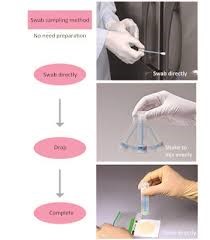
Swab Sampling of Internal Surfaces:
-
- Used to assess microbial contamination within air lines or nozzles.
- Common Media Used
- Nutrient Agar: General-purpose medium for bacteria.
- Sabouraud Dextrose Agar (SDA): Selective for fungi and yeasts.
- Culture Media & Incubation Conditions
- Typically, at 30–35°C for 3–5 days for bacteria, and 20–25°C for 5–7 days for fungi. Or We also provide custom incubation or media options
- depending on your industry or regulatory requirements
-
How to Minimize Microbial Contamination
Here are proactive steps every facility should take:
- ✅ Use sterile point-of-use filters
- ✅ Keep moisture traps and desiccant dryers well maintained
- ✅ Design systems without dead legs
- ✅ Regularly clean and validate compressed air pipelines
- ✅ Train technicians in aseptic maintenance procedures
💡 Regular microbial monitoring ensures your system is working as intended — and avoids expensive surprises during audits.
-
Why Choose SWA Enviro for Compressed Air Testing?
We’re more than just a testing lab — we’re your compliance partner.
Our Services Include:
- ✔️ On-site sampling across India
- ✔️ ISO 8573-7 & GMP-compliant reports
- ✔️ Advanced microbial air samplers & equipment
- ✔️ Detailed validation documentation for audits
- ✔️ Quick turnaround from our NABL-accredited laboratory
-
Conclusion
Don’t let compressed air become your weakest link.
Whether you’re in pharmaceuticals, food, biotech, or cleanroom manufacturing, microbial testing of compressed air is essential. At SWA Enviro, we help you stay compliant, safe, and audit-ready — all while protecting your brand and end users.
Make microbial air quality a core part of your quality management system.

